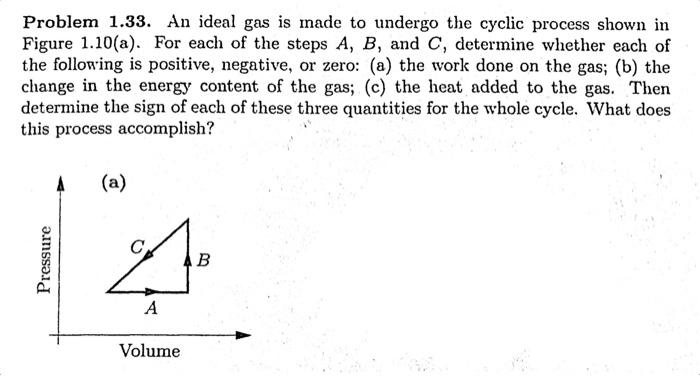
An Ideal Gas Is Made to Undergo the Cyclic Process
For each of the steps A B and C determine whether each of the following is positive negative or zero. An ideal gas is made to undergo the cyclic process shown in Figure 110a.

An Ideal Gas Is Made To Go Through A Cyclic Thermodynamical Process In Four Steps The Amount Of Heat Involved Are Q1 600 J Q2 400 J Q3 300
One mole of a mono-atomic ideal gas is made to undergo a quasi-static cyclic process.
. For each of the steps A B and C determine whether each of the following is positive negative or zero. An ideal gas is made to undergo the cyclic process shown in Figure 110a. What Is Heat Engine.
C the heat added to the gas. If the cycle goes anticlockwise then the work is done on the system every cycle. B -2P 0 V 0.
An ideal gas is made to undergo a cycle depicted by the p -V. For each of the steps A B and C determine whether each of the following is positive nogative or zero. PV γ Constant C P Pressure VVolume γratio of specific heat Given.
C the heat added to the gas. C the heat added to the gas. C 2 P 0 V o.
B the change in the energy content of the gas. An example of such a system is a refrigerator or air conditioner. An ideal gas undergoes a cyclic process as shown in Figure.
The curved line from A to B is an adiabat. The curved line from A to B is an adiabat Then- A The efficiency of this cycle is given by unity as no heat is released during the cycle B Heat is absorbed in the upper part of the straight line path and released in the lower part. PV nRT Where R Universal gas constant TTemperature n Number of moles U 52 nRT C Change in internal energy.
Sign of each of these three quantities for the whole cycle will be 0 refers to no change. B 0. A the work done on the gas.
C the heat added to the gas. A heat engine is a device by which a system is made to undergo a cyclic process that results in the conversion of heat. A the work done on the gas.
C the heat added to the gas. Given C v for gas 15 R 4-13 063. B the change in the energy content added to the gas.
One mole of an ideal monatomic gas undergoes a process described by the equation PV3constantThe heat capacity of the gas during this process is. For each of the steps A B C determine whether each of the following is positive negative or zero. Then determine the sign of each of these three.
C the heat added to the gas. B the change in the energy content of the gas. Sign of each of these three quantities for the whole cycle will be 0 refers to no change A 0.
Asked Mar 4 2018 in Class XI Physics by vijay Expert 79k points An ideal gas undergoes cyclic process ABCDA as shown in given P-V diagram. An ideal gas is made to undergo a cycle depicted by the p V diagram given below. Determine the net work involved in this cyclic process in term of R.
C the heat added to the gas. An ideal gas is made to undergo the cyclic process shown in Figure 110a. An ideal gas is made to undergo the cyclic process shown in Figure 110 a.
An ideal gas is made to undergo a cycle depicted by the PV diagram alongside. C the heat added to the gas. An ideal gas is made to undergo the cyclic process shown in the figure below.
In the last final step gas was compressed reversibly and adiabatically to initial state. C 0 0 0. B the change in the energy content of the gas.
The Carnot engine is the best example of a cyclic process. For each of the steps A B and C determine whether each of the following is positive negative or zero. B the change in the energy content of the gas.
Let ΔW depict the work done. C the heat added to the gas. A the work done on the gas.
During the process A--B temperature T of the gas varies with its volume V according to the equation T bV2 where b is a constant. An ideal gas is made to undergo a cyclic process shown in the figure to the right. The amount of work done by the gas is.
B the change in the energy content of the gas. Then determine the sign of each of these three quantities for the whole. During the whole cycle maximum pressure of the gas is two times of the minimum pressure.
B the change in the energy content added to the gas. An ideal gas is made to undergo the cyclic process shown in the figure below. An ideal gas is made to undergo a cyclic process shown in the figure to the right.
Δ UBC - 5 kJ mol-1 qAB 2 kJ mol-1 WAB - 5 kJ mol-1 WCA 3 kJ mol-1 Heat absorbed by the system during process CA is. A the work done on the gas. Then determine the sign of each of these three quantities for the whole cycle.
A 6P g V 0. A the work done on the gas. For each of the steps A B and C determine whether each of the following is positive negative or zero.
D 4P o V 0. Then the gas is made to undergo an isochoric process in which its temperature is found to decrease. Two moles of helium gas undergoes a cyclic process as shown in the figure.
U 5PV2 C For an ideal gas. An ideal gas is made to undergo the cyclic process shown in Figure 110 a. An ideal gas is made to undergo the cyclic process shown in Figure 110mathrma For each of the steps A B and C determine whether each of the following is positive negative or zero.
ΔU be the change in internal energy of the gas and Q be the heat added to the gas. A the work done on the gas. Asked by aayush 7th March 2018 734 PM.
For each of the steps A B C determine whether each of the following is positive negative or zero. Then 1467 65 KVPY KVPY 2010 Thermodynamics Report Error. B the change in the energy content of the gas.
Let Δ W depict the work done Δ U be the change in internal energy of the gas and Q be the heat added to the gas. The equation of adiabatic process in PV plane is. The question is one mole of an ideal monoatomic gas is caused to go through a cyclic as shown in the figure the internal energy of the gas from a to b and b2c effectively internet it is given by Delta u is equal to 82 B2 B2 - fever we were there.
A the work done on the gas. ΔU nf R ΔT22. A the work done on the gas.
An ideal gas is made to undergo the cyclic process shown in Figure. For each of the steps A B and C determine whether each of the following is positive negative or zero. Then determine the sign of each of these three quantities for the whole cycle.
B the change in the energy content of the ga. ΔU 52nR ΔT1 We know. Assuming ideal behaviour of gas the net work done by the gas in this cyclic process is a overline0 b 100 R ln 2 c 100 R ln 4 d 200 R ln 4.
A the work done on the gas. Then determine the sign of each of these three quantities for the whole. Then determine the sign of each of these three.

N Moles Of A Perfect Gas Undergoes A Cyclic Process Abca Consisting Of
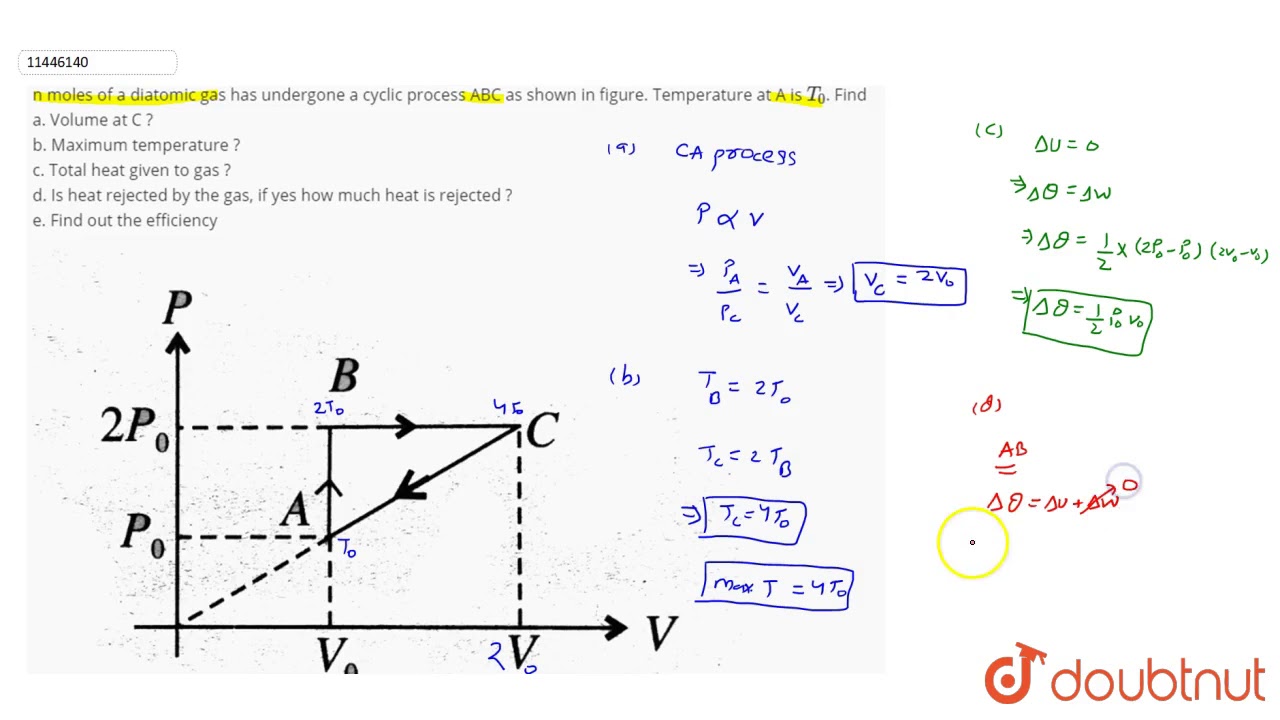
N Moles Of A Diatomic Gas Has Undergone A Cyclic Process Abc As Shown In Figure Temperature At Youtube

An Ideal Monoatomic Gas Undergoes A Cyclic Process Abca As Shown In The Fig The Ratio Of Heat Absorbed During Ab To The Work Done On The Gas During Bc Is
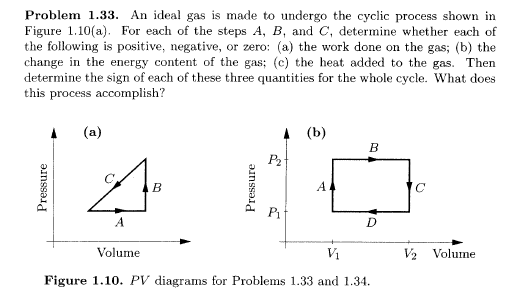
Solved Problem 1 33 An Ideal Gas Is Made To Undergo The Chegg Com

Two Moles Of Helium Gas Undergo A Cyclic Process As Shown In Figure Assuming The Gas To Be Ideal Calculate The Following Quantities In This Process The Net Change In The Heat
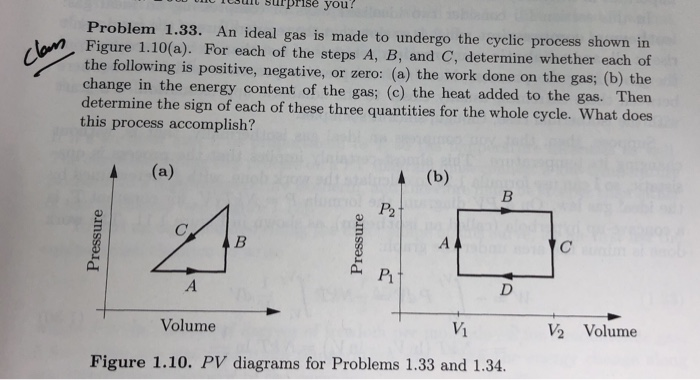
Solved Rprise You Problem 1 33 An Ideal Gas Is Made To Chegg Com
An Ideal Gas Is Made To Undergo The Cyclic Process Shown In The Figure Below Let Dw Depict The Work Done Du Be The Change In Internal Energy Sarthaks Econnect

One Mole Of An Ideal Monoatomic Gas Is Taken Round Cyclic Process Abca As Shown In Figure Calculate A The Work Done By The Gas B The Heat Rejected By The Gas In

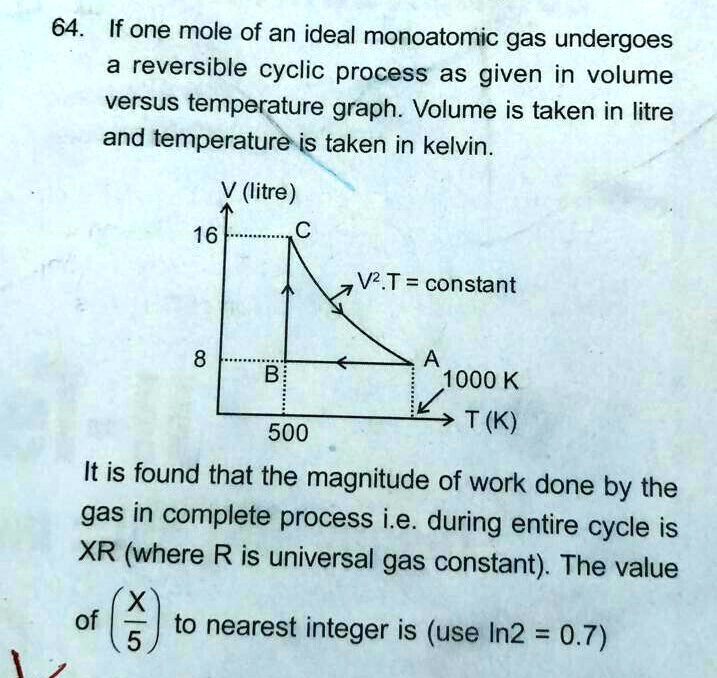
Solved 64 If One Mole Of An Ideal Monoatomic Gas Undergoes A Reversible Cyclic Process As Given In Volume Versus Temperature Graph Volume Is Taken In Litre And Temperature Is Taken In Kelvin

Two Moles Of Helium Gas Undergo A Cyclic Process As Shown In Figure Assuming The Gas To Be Ideal Calculate The Following Quantities In This Process The Net Change In The Heat
Solved Problem 1 33 An Ideal Gas Is Made To Undergo The Chegg Com

Solved An Ideal Gas Is Made To Undergo The Cyclic Process Shown In The Figure For Each Of The Steps A B And C Determine Whether Each Of The Following Positive Negative
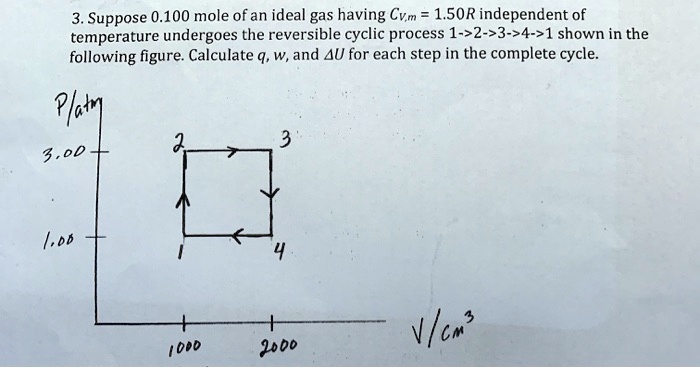
Solved 3 Suppose 0 100 Mole Ofan Ideal Gas Having Cvm 1 50r Independent Of Temperature Undergoes The Reversible Cyclic Process 1 2 3 24 1 Shown In The Following Figure Calculate 9 W And Au For Each Step In

Two Moles Of Helium Gas Undergo A Cyclic Process As Shown In Fig Assuming The Gas To Be Ideal Youtube

Thermo Thursday Chapter 1 5 Compression Work Continued Eugene Fischer
An Ideal Gas Is Taken A Cyclic Thermodynamic Process Through Four Steps The Amount Of Heat Involved Sarthaks Econnect Largest Online Education Community
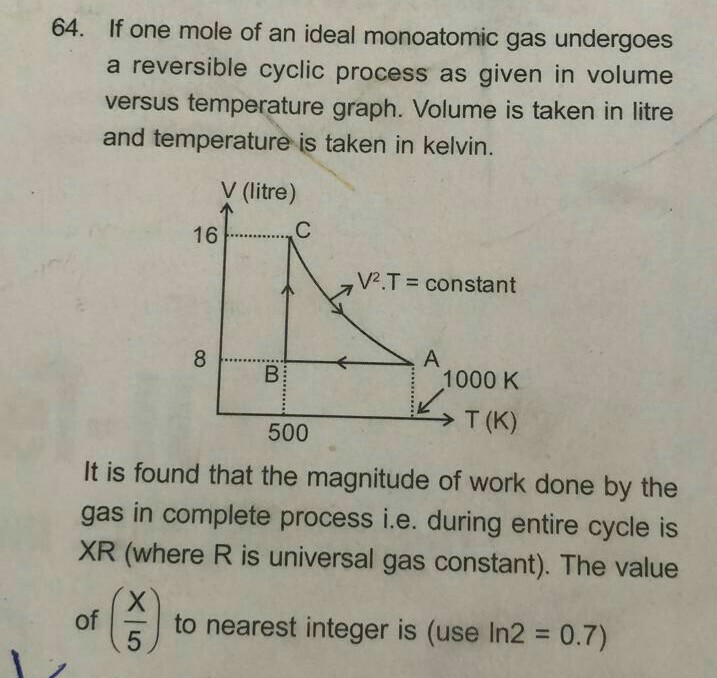
Solved 64 If One Mole Of An Ideal Monoatomic Gas Undergoes Chegg Com
Two Moles Of Helium Gas Undergo A Cyclic Process As Shown In The Figure Assuming The Gas To Be Ideal Sarthaks Econnect Largest Online Education Community

An Ideal Gas Is Made To Undergo The Cyclic Process Shown In The Figure Below Let W Youtube
Comments
Post a Comment